LNP™ COMPOUNDS – ELECTRICALLY CONDUCTIVE SOLUTIONS FOR THE HEALTHCARE INDUSTRY
In the Healthcare Industry over the last decades there has been a continued search for material solutions, that are addressing the macro trends in this industry of more regulatory oversight, cleaner and safer devices, non threatening, reduced patient anxiety operations, efficient, more personalized medical devices, that are portable, small and lightweight and last but not least, also more environmentally sustainable solutions.
PLASTICS IN MEDICAL DEVICES
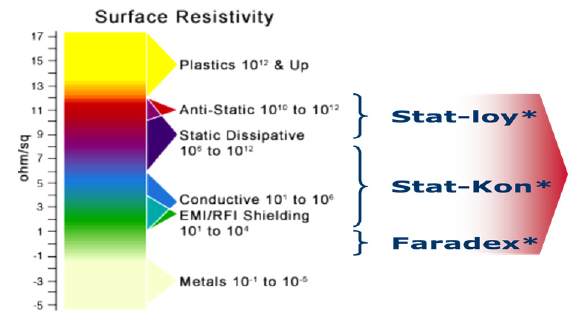
When operating with standard (non-electrically conductive) plastics in medical devices there are 2 main challenges to be addressed. 1) In drug delivery applications, the issue of inconsistent dosing and dust, particle attraction & contamination can occur due to electrostatic effects and 2) Malfunctioning of critical supporting electronic devices, due to electromagnetic interference.
ELECTRICALLY CONDUCTIVE COMPOUNDS
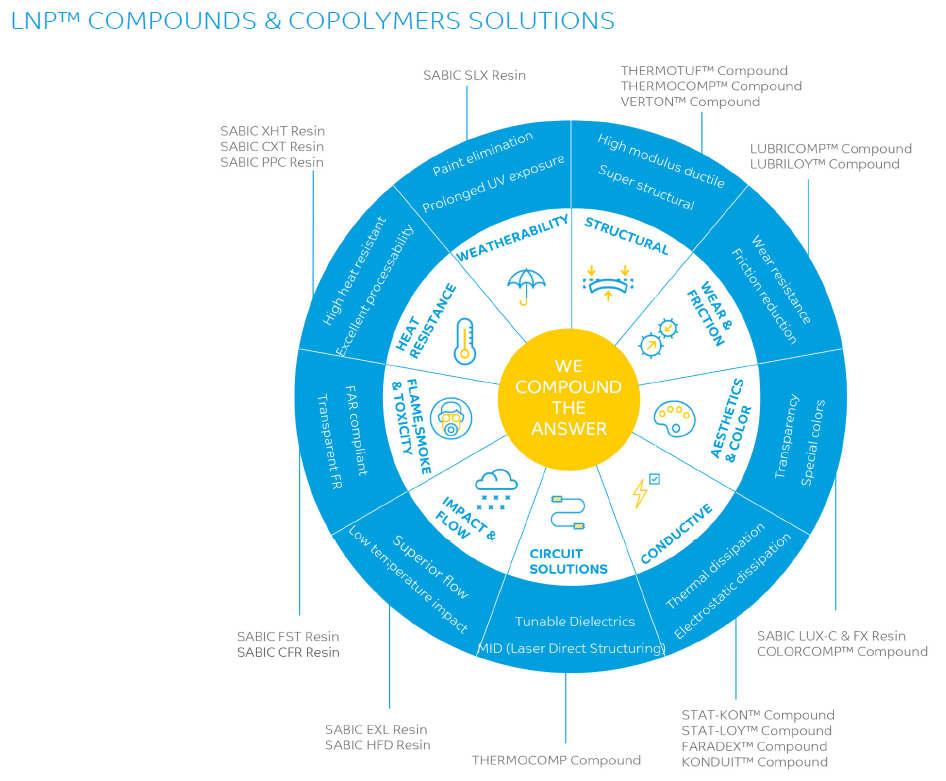
The addition of a conductive filler to a thermoplastic material can increase the electrical conductivity and/or the EMI shielding capability of a thermoplastic, herewith reducing the aforementioned risks. Traditional fillers like carbon powder, fibers, anti-stat agents and stainless-steel fibers are effective, and more novel and advanced technologies can provide additional features. The resulting conductive compounds do exhibit a better carbon footprint versus plastics with a conductive paint and metals.
SABIC’S HEALTHCARE POLICY
As a means to support the Healthcare Industry in mitigating risks, SABIC’s LNP business has developed a HC Policy:
• Easily identifiable "healthcare product" nomenclature
• Biocompatibility assessed (according to ISO10993 or USP Class VI)
• Food contact compliance for most healthcare products
• FDA Drug Master File and/or device master file listing (letter of authorization provided as needed)
• SABIC healthcare products are subject to formula lock and stringent management of change process
• SABIC healthcare products are being manufactured under GMP rules (No.2023/2006(Commission Regulation EC, 22 December 2006) or FDA21CFR174.5).
• Long-term supply options available
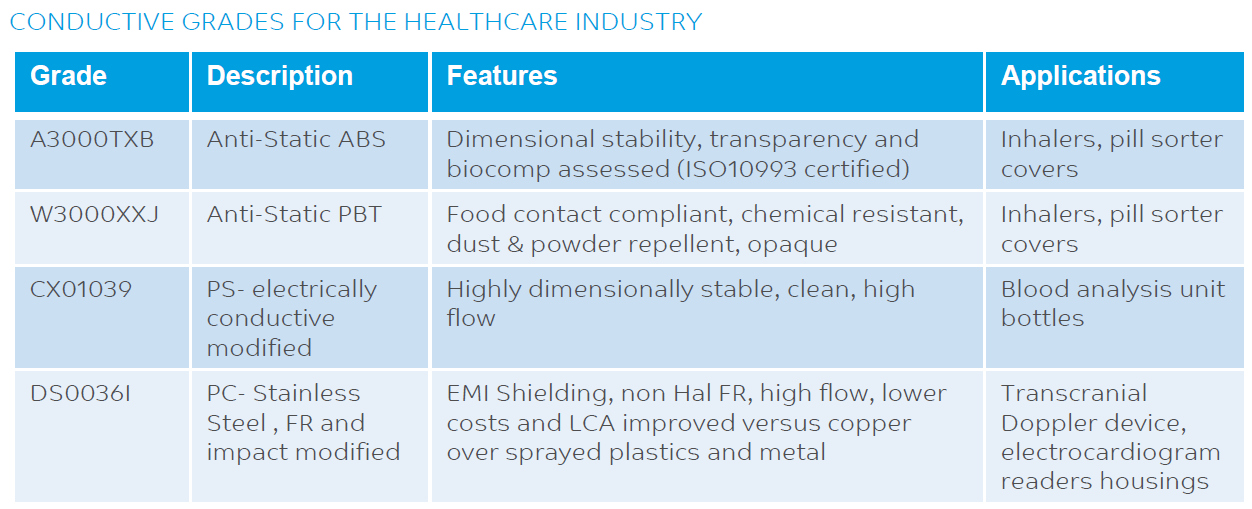
PRODUCTS AND GRADES
CONTACT US
Reach out to us for one-on-one support to ensure you have all the information and insights you need to choose the best-fit material for your application.
DISCLAIMER: THE MATERIALS. PRODUCTS AND SERVICES OF CORPORATION OR ITS
SUBSIDIARIES OR AFFILIATES (“SELLER”) ARE SOLD SUBJECT TO SELLER'S STANDARD CONDITIONS OF SALE WHICH ARE AVAILABLE UPON REQUEST INFORMATION AND RECOMMENDATIONS CONTAINED IN THIS DOCUMENT ARE GIVEN IN GOOD FAITH. HOWEVER SELLER MAKES NO EXPRESS OR IMPLIED REPRESENTATION, WARRANTY OR GUARANTEE
(i) THAT ANY RESULTS DESCRIBED IN THIS DOCUMENT WILL BE OBTAINED UNDER END-USE CONDITIONS. OR (ii) AS TO THE EFFECTIVENESS OR SAFETY OF ANY DESIGN OR APPLICATION INCORPORATING SELLER'S MATERIALS, PRODUCTS, SERVICES OR RECOMMENDATIONS UNLESS OTHERWISE PROVIDED IN SELLER'S STANDARD CONDITIONS OF SALE, SELLER SHALL NOT BE RESPONSIBLE FOR ANY LOSS RESULTING FROM ANY USE OF ITS MATERLALS, PRODUCTS, SERVICES OR RECOMMENDATIONS DESCRIBED IN THIS DOCUMENT. Each user is responsible for making its own determination as to the suitability of Seller’s materials, poducts, services or recommendations for the user’s particular use through appropriate end-use testing and analysis. Nothing in any document or oral statement shall be deemed to alter or waive any provision of Seller’s Standard conditions of Sale of this Disclaimer, unless it is specifically agreed to in a writing signed by Seller. Statements by Seller concerning a possible use of any material, product, service or design so not are not intended to and should not be construed to grant any ‘license under any patent or intellectual property right of Seller or as a recommendation for the use of any material, product, service or design in a manner that infringes any patent or other intellectual property right.
SABIC and brands marked with TM are trademarks of SABIC or its subsidiaries or affiliates, unless otherwise noted. © 2023 Saudi Basic Industries Corporation (SABIC). All rights reserved. Any brands, products or services of other companies referenced in this document are the trademarks, service marks and/or trade names of their respective holders.
Your PDF is downloading. Please wait.
Register for High value Multi-Point Data
- Access validated product data to streamline material selection
- Get inspired by innovative application ideas
- Register for high value multi-point data and a personalized experience

