ULTEM™ HU1004 RESIN- A HIGH PERFORMANCE RESIN BLEND FOR MULTIPLE STERILIZATION ENVIRONMENTS
INTRODUCTION
HARSH STERILIZATION ENVIRONMENTSMedical devices and the materials used to construct them must withstand ever increasing in-service demands due to more complex designs, performance requirements and regulations. In particular, the heightened emphasis on infection control is intensifying the spotlight being shined on the range of processes used to sterilize medical and dental equipment and devices. And trends in the industry are pointing towards designing more inherent functional capabilities into reusable devices, meaning that they are more likely to contain sensitive electronics (vulnerable to heat and moisture) or other features which could require different sterilization options.
Materials must now be able to withstand a number of different potential sterilization processes –such as high temperature steam autoclave (up to 134 °C), gamma irradiation, and an emerging process suitable at low temperatures: hydrogen peroxide gas sterilization. All of these sterilization methods have the potential to degrade devices over time, diminishing their mechanical integrity, interfering with performance, or altering their aesthetics.
REQUIRE A HIGH-PERFORMANCE SOLUTION
Among the family of high-performance engineered thermoplastics offered by SABIC to help healthcare device manufacturers meet these challenges are ULTEM resins, which have proven their capability in each sterilization environment. In particular, SABIC’s innovative ULTEM HU1004 resin, a polyetherimide (PEI) resin blend with enhanced hydrolytic stability, has been developed specifically for healthcare applications. This proprietary, patent‑pending blend of PEI and another high-performance polymer provides synergistic advantages that the individual polymers alone cannot provide while maintaining inherent transparency.

ULTEM HU1004 RESIN: A PROVEN PERFORMER
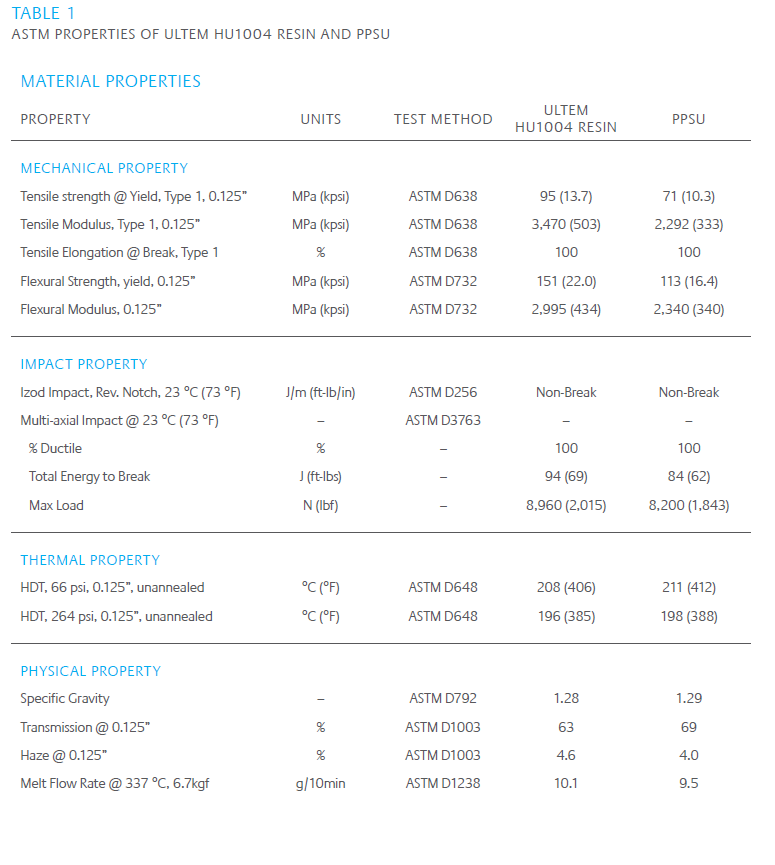
ULTEM HU1004 resin offers medical device designers and manufacturers a single material solution for the healthcare industry since it is capable of withstanding rigorous sterilization processes such as steam sterilization at 134 °C, gamma irradiation or hydrogen peroxide vapor sterilization (with or without plasma). Additionally, ULTEM HU1004 resin has agency approval of the FDA, biocompatibility (ISO 10993 or USP Class VI) and EUFC.
SUPERIOR PERFORMANCE
SUPERIOR PERFORMANCE IN HYDROGEN PEROXIDE PLASMA STERILIZATION SYSTEMS
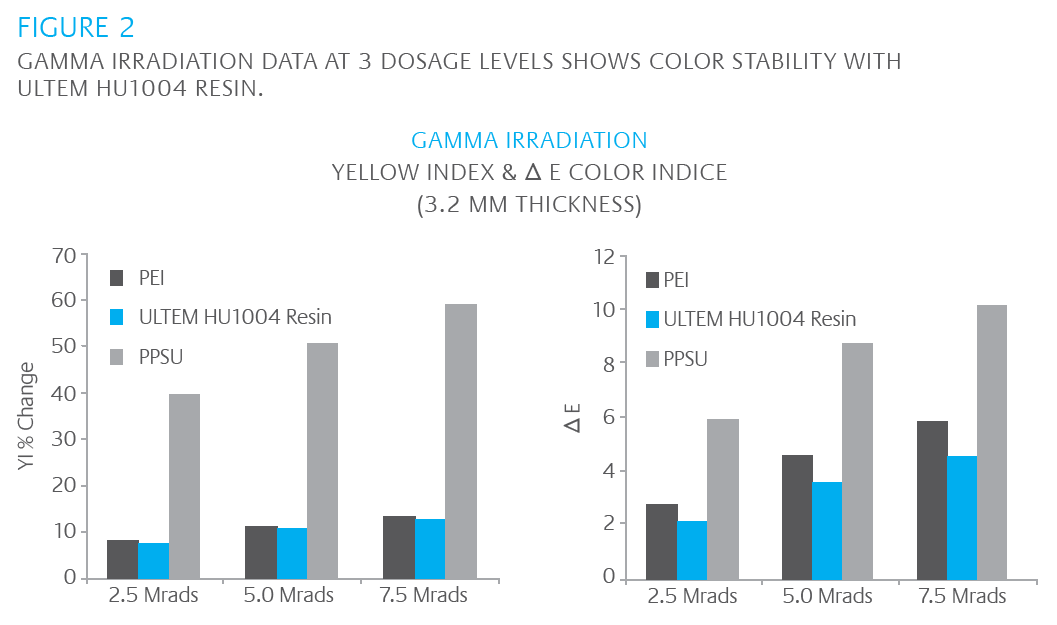
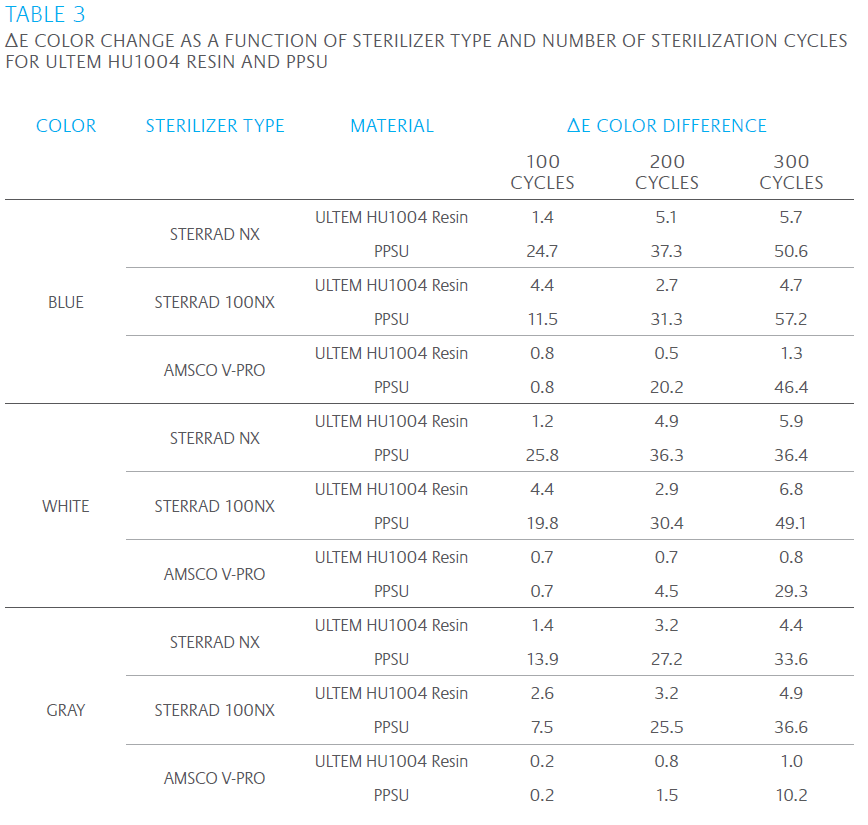
Our research has shown that ULTEM HU1004 resin maintains tensile strength and ductility – important to extending the useful life of devices – in hydrogen peroxide plasma sterilization systems, outperforming a competitive material, polyphenylsulfone (PPSU). The same study also demonstrated that ULTEM HU1004 resin has a superior ability to retain color and appearance. The study tested the performance of both ULTEM HU1004 resin and PPSU in at least 300 sterilization cycles in STERRAD† NX†, STERRAD 100NX† plasma and AMS CO† V-PRO† vapor systems. Data shows ULTEM HU1004 resin significantly outperforming PPSU. The results are compiled in the Tables 2 and 3 and Figures 2 – 4 where retention of ductility, in particular, and color stability are clearly demonstrated versus competitive materials.

COLOR STABILITY

COLOR ME....STABLE!


MAKE THE BEST CHOICE
With a broad portfolio of more than 50 healthcare products which adhere to SABIC’s stringent healthcare product policy, we offer medical device designers and manufacturers a number of options when considering a balance between properties, performance and methods of sterilization.
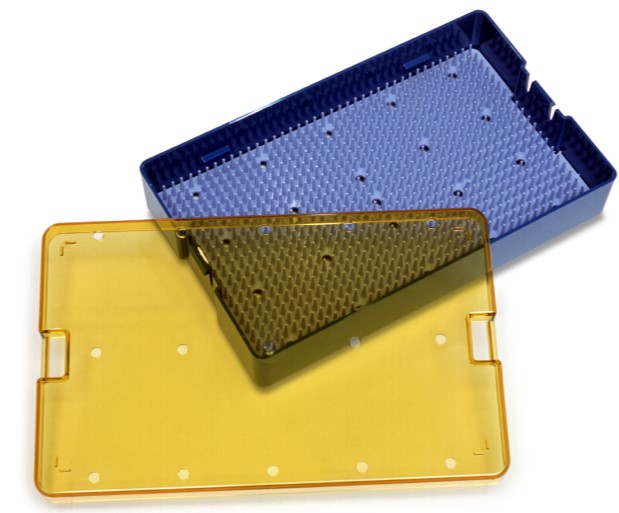
Indusbello Sterilization Tray
To address the growing healthcare trend of healthcare associated infections, SABI C has worked closely with Indusbello to develop a differentiated solution for sterilization trays to help improve the safety of patients and clinician healthcare environments. SABIC’s ULTEM HU1004 resin is designed to withstand a number of different harsh sterilization processes, including high temperature steam autoclave (up to 134 °C), gamma radiation and an emerging process suitable at low temperatures - hydrogen peroxide gas sterilization. In addition, the material can provide flexibility in design, transparency, superior aesthetics and enhanced productivity through injection molding.
ULTEM HU1004 resin has been pre-assessed for biocompatibility per ISO 10993. The product passed the following tests:
• ISO 10993 Part 5 Tests for In Vitro Cytotoxicity (L929 Neutral Red Uptake)
• ISO 10993 Part 6 Tests for Local Effects After Implantation (2-wk Intramuscular Implantation)
• ISO 10993 Part 10 Tests for Irritation and Skin Sensitization (Kligman Maximization and Intracutaneous Injection)
• ISO 10993 Part 11 Tests for Systemic Toxicity (Systemic Injection and Rabbit Pyrogen)
• ASTM F756-08 Standard Practice for Assessment of Hemolytic Properties of Materials (Hemolysis - Rabbit Blood)
• United States Pharmacopeia 35 Monograph <661> Containers, Physicochemical Tests (Non-Volatile Residue)
• United States Pharmacopeia 35 Monograph <662> Containers, Physicochemical Tests
SABIC does not recommend and will not support the use of any SABIC products in medical devices intended to remain continuously in the human body for longer than 29 days. The customer is in the best position to know the details of the intended conditions of use of their product. It is incumbent on them to carry out the appropriate biocompatibility tests of their product to assure safety, efficacy, and regulatory compliance. SABIC considers the determination of suitability of ULTEM HU1004 resin for a medical device to be the responsibility of the device manufacturer and the drug packager.
To assist in the review of the use of ULTEM HU1004 resin for medical devices and drug packages with the appropriate FDA regulatory personnel, we maintain a Drug Master File (DMF-1562) and a Device Master File (MAF-91) with the FDA. The DMF / MAF contain detailed formulation information and test data on certain grades of ULTEM resins. SABIC considers this information proprietary and does not divulge it without a properly executed secrecy agreement. The FDA holds this information in confidence, but with our specific authorization will review it on behalf of a specific company’s application or submittal for the purpose of rendering an opinion about the safety and suitability of the proposed usage.

PRODUCTS AND GRADES
CONTACT US
Reach out to us for one-on-one support to ensure you have all the information and insights you need to choose the best-fit material for your application.
DISCLAIMER: THE MATERIALS, PRODUCTS AND SERVICES OF SAUDI BASIC INDUSTRIES CORPORATION (SABIC) OR ITS SUBSIDIARIES OR AFFILIATES ("SELLER") ARE SOLD SUBJECT TO SELLER'S STANDARD CONDITIONS OF SALE, WHICH ARE AVAILABLE UPON REQUEST. INFORMATION AND RECOMMENDATIONS CONTAINED IN THIS DOCUMENT ARE GIVEN IN GOOD FAITH. HOWEVER, SELLER MAKES NO EXPRESS OR IMPLIED REPRESENTATION, WARRANTY OR GUARANTEE (i) THAT ANY RESULTS DESCRIBED IN THIS DOCUMENT WILL BE OBTAINED UNDER END-USE CONDITIONS, OR (ii) AS TO THE EFFECTIVENESS OR SAFETY OF ANY DESIGN OR APPLICATION INCORPORATING SELLER'S MATERIALS, PRODUCTS, SERVICES OR RECOMMENDATIONS.UNLESS OTHERWISE PROVIDED IN SELLER'S STANDARD CONDITIONS OF SALE, SELLER, SHALL NOT BE RESPONSIBLE FOR ANY LOSS RESULTING FROM ANY USE OF ITS MATERIALS, PRODUCTS, SERVICES OR RECOMMENDATIONS DESCRIBED IN THIS DOCUMENT. Each user is responsible for making its own determination as to the suitability of Seller's materials, products, services or recommendations for the user's particular use through appropriate end-use and other testing and analysis. Nothing in any document or oral statement shall be deemed to alter or waive any provision of Seller's Standard Conditions of Sale or this Disclaimer, unless it is specifically agreed to in a writing signed by seller. Statements by Seller concerning a possible use of any material, product, service or design do not, are not intended to, and should not be construed to grant any license under any patent or other intellectual property right of Seller or as a recommendation for the use of any material, product, service or design in a manner that infringes any patent or other intellectual property right.
SABIC and brands marked with ™ are trademarks of SABIC or its subsidiaries or affiliates, unless otherwise noted © 2023 Saudi Basic Industries Corporation (SABIC). All Rights Reserved. Any brands, products or services of other companies referenced in this document are the trademarks, service marks and/or trade names of their respective holders.
Your PDF is downloading. Please wait.
Register for High value Multi-Point Data
- Access validated product data to streamline material selection
- Get inspired by innovative application ideas
- Register for high value multi-point data and a personalized experience

